Focus on diagnostics
We process the data
NGS panels

Whole exome sequencing
Cancer diagnostics
Solution
The varvis® genomics platform is a complete solution for clinical diagnostics, supporting NGS raw data processing, genomics data management, and variant interpretation. Automated CNV and SNV analysis are clinically validated and completely integrated into the NGS workflow.
Bioinformatics
Automated. Validated. Fast.
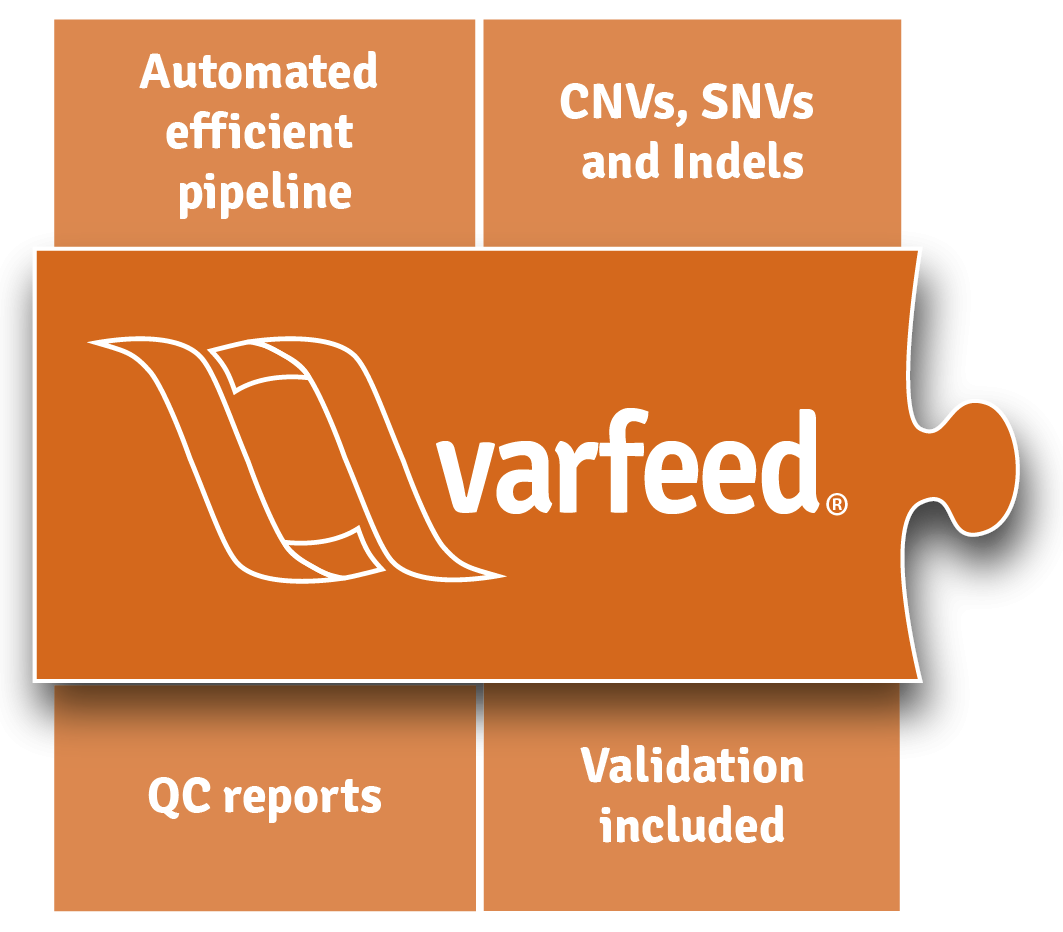
varfeed® automates the processing of raw next-generation sequencing data from bcl, fastq or bam files. It performs all bioinformatics, including alignment and clinically validated CNV/SNV/Indel variant calling. All results are handed over to varvis® automatically. Pipeline validation is provided as a service – updates are included.
varvis® genomics software
Made for use in clinical diagnostics
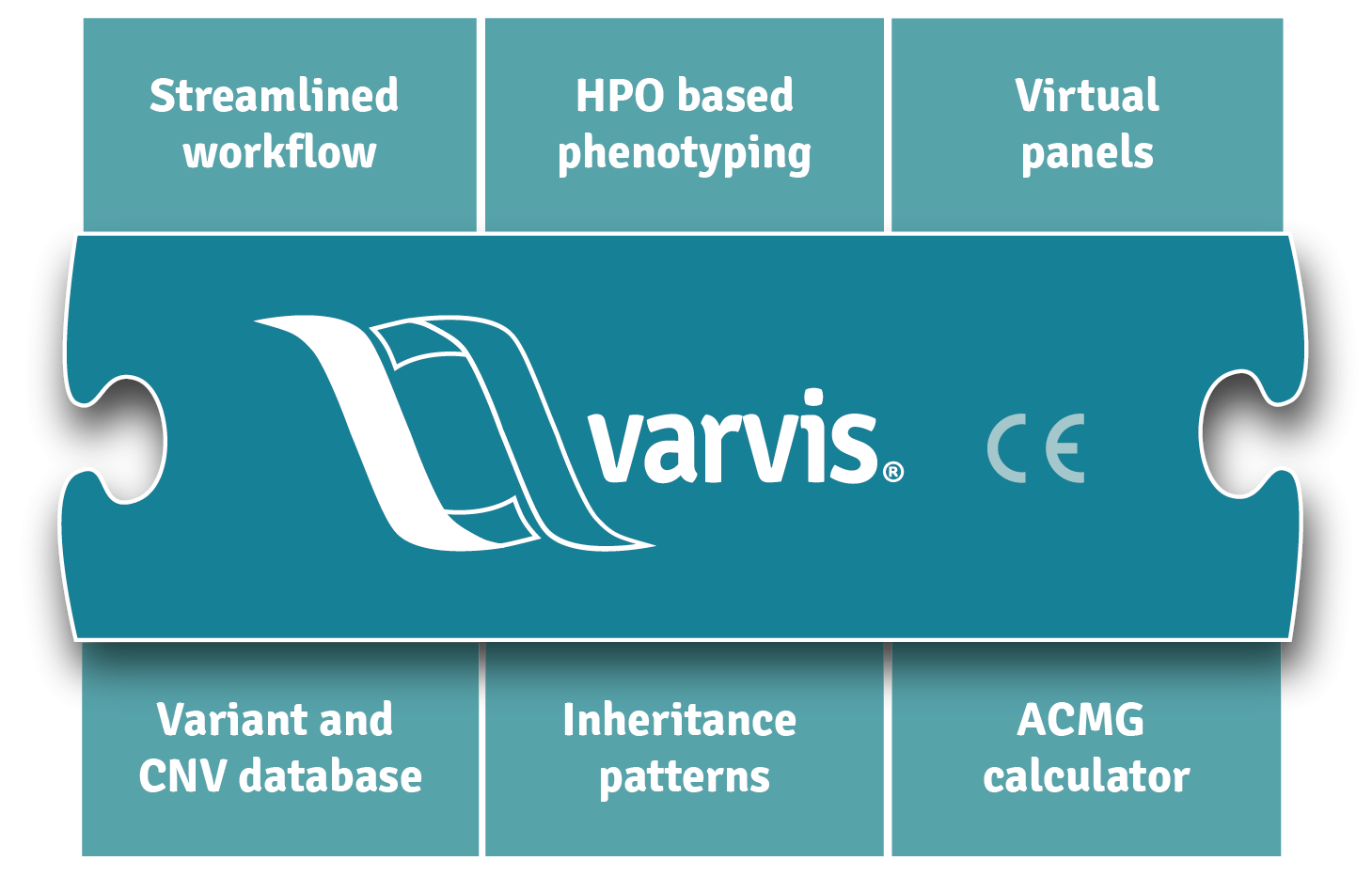
varvis® is a clinical decision support system (CDS) and allows you to review, filter, and classify genetic variants. In addition, varvis® is your own comprehensive variant database. It supports the clinical decision-making process and is a registered CE-IVD device according to directive 98/79/EC.
Annotation
Always up to date
allexes® provides the data for variant annotation to varvis®. allexes® does not only deliver the most recent versions of public databases, but also provides access to aggregated genomic reference data from all our users compliant with HIPAA and EU regulations.
Our Key Benefits
Our Instructors
Irene Patric
Dr. Yvonne Kasmann

